CT Chest (Thorax) - CAM 750
GENERAL INFORMATION
- It is an expectation that all patients receive care/services from a licensed clinician. All appropriate supporting documentation, including recent pertinent office visit notes, laboratory data, and results of any special testing must be provided. If applicable: All prior relevant imaging results and the reason that alternative imaging cannot be performed must be included in the documentation submitted.
- Where a specific clinical indication is not directly addressed in this guideline, medical necessity determination will be made based on widely accepted standard of care criteria. These criteria are supported by evidence-based or peer-reviewed sources such as medical literature, societal guidelines and state/national recommendations.
- The guideline criteria in the following sections were developed utilizing evidence-based and peer-reviewed resources from medical publications and societal organization guidelines as well as from widely accepted standard of care, best practice recommendations.
Policy
Chest Computed Tomography (CT) generates images of the organs and structures in the chest (thorax) with the use of radiation.
INDICATIONS FOR CHEST CT
Follow-Up of Lung Nodules
NOTE: LUNG-RADS table is used to follow-up on lung nodules on low dose CT (LDCT) that will be followed up with a low dose CT. The Fleischner table is used to follow-up on incidental lung nodules seen on studies other than a low dose CT (such as Chest x-ray or Chest CT). If multiple nodules, the largest and most concerning is used for the decision.
Lung Nodules With No History of Malignancy(1,2)
- Incidental pulmonary nodule on x-ray:
- Immediate follow up with Chest CT if the nodule is indeterminate as per the radiology report (i.e., not typical of granulomatous disease)
- Incidental pulmonary nodules on non-chest CT (such as a neck, shoulder or abdomen CT):
- Nodules > 8 mm or those with very suspicious features need a dedicated Chest CT as early as possible
- Nodules ≤ 8 mm should follow the Fleischner table (see Table 2) to determine when a dedicated Chest CT is needed
- Follow-up of pulmonary nodules detected on a non-screening (regular) Chest CT is as per Fleischner table (see Table 2) when ALL of the following criteria are met:
- Individual is ≥ 35 years old
- If age is < 35, non-malignant causes are more likely, and infection should be excluded. Once infection is excluded if the nodule is unable to followed with chest x-ray and explanation given as to how management would change, coverage can be considered on a case-by-case basis
- No personal history of cancer
- If there is a prior history of cancer, follow-up imaging can be done as soon as 3 months
- No immunocompromise
- If the patient is immunocompromised, follow-up imaging can be done as soon as 1 month if there is suspicion of fulminant infection
- Individual is ≥ 35 years old
Follow-Up of Lung Nodule Seen on Screening LDCT
Follow-up of pulmonary nodule identified on screening LDCT (for patients at high risk for lung cancer) as per Lung-RADS criteria (3) (see Table 1 - typically ordered as a LDCT))
Infection and Inflammation
Infection Follow-Up Imaging
- Recent pneumonia and suspected parapneumonic effusion or empyema after recent chest x-ray(4)
- For evaluation of non-resolving pneumonia or inflammatory disease documented by at least two imaging studies:
- Unimproved with 4 weeks of antibiotic treatment; OR
- Unresolved at 8 weeks(5)
Diffuse Lung Disease (DLD)
- For evaluation of suspected DLD (including diffuse parenchymal and interstitial lung diseases) after initial chest x-ray excludes a more acute disease (such as pneumonia) in the following situations (6):
- Based on restrictive pattern pulmonary function test
- In patients with known collagen vascular disease in whom DLD is suspected
- For biopsy guidance when DLD is suspected (7)
- With signs or symptoms unresponsive to treatment such as:
- Shortness of breath
- Persistent dyspnea
- Persistent cough
- For reassessment of known DLD
- Annually OR
- For clinical progression of disease
NOTE: Chest CT for evaluation of DLD is typically performed with a high-resolution protocol (HRCT)
Sarcoidosis8
- For suspected sarcoidosis after initial workup including chest X-ray
- For known sarcoidosis when there are EITHER symptoms of progression (including normal CXR with unexplained dyspnea or cough) OR progression on chest X-ray
Granulomatosis with Polyangiitis (GPA) (Formally Wegener’s Granulomatosis)(9)
- Advanced imaging for GPA is indicated with any ONE of the following:
- Suspected GPA based on clinical findings (such as biopsy results, lab testing including antineutrophil cytoplasmic antibodies (ANCA))
- Known GPA when imaging results of a specific anatomic area are needed to guide systemic therapy
NOTE: Imaging of the Sinus, Neck and/or Abdomen may also be indicated for GPA as involvement of the airway, lungs, and/or kidneys is common
Tuberculosis (TB)10
Known or suspected tuberculosis and initial chest X-ray completed
Esophagus / Pharynx
- Indicated for the evaluation of dysphagia after appropriate prior work up including endoscopy (EGD) and/or fluoroscopic studies (such as modified barium swallow, biphasic esophogram) is indeterminate or abnormal(11)
Chronic Cough (16)
- Symptoms > 8 weeks and chest x-ray completed and any of the following:
- Clinical concern for bronchiectasis
- After evaluation for other causes and failed treatment for those diagnosed with:
- Asthma
- Gastroesophageal Reflux Disease
- Discontinuation of ACE inhibitors
- Postnasal drip
Thoracic Aortic Disease(13–16)
Screening for possible Thoracic Aortic Aneurysm (TAA)
- Screening in individuals with a personal history of bicuspid aortic valve when prior TTE (Transthoracic Echocardiogram) is indeterminate or abnormal:
NOTE: Typical TTE follow-up imaging intervals for bicuspid aortic valve patients:
- Baseline study at initial diagnosis of bicuspid aortic valve
- Follow-up imaging is based on findings on prior imaging of a dilated aorta of > 40 mm is typically every 2-3 years thereafter
- Screening in individuals at elevated risk due to family history when TTE (Transthoracic Echocardiogram) is inconclusive or insufficient as clinically indicated with any ONE of the following (14):
- First-degree relatives of individuals with a known aortic dissection OR known thoracic aortic aneurysm (defined as 1.5 times (> 50%) larger than the predicted aorta size based on age, sex, body size)
- First and/or second-degree relatives of individuals with heritable thoracic aorta disease (HTAD) (HTAD comprises a clinically and genetically heterogeneous group of disorders sharing the common denominator of aneurysm or dissection of the thoracic aorta)
NOTE: previous analogous terminology includes familial thoracic aortic aneurysm and dissection (FTAAD) and non-syndromic heritable thoracic aortic disease (NS-TAD))
- First degree relatives of individuals with a known bicuspid aortic valve
- See Imaging in Known Genetic Conditions section for additional indications for screening
Thoracic Aortic Aneurysm (TAA)
- Asymptomatic known or suspected thoracic aortic aneurysm
- With prior indeterminate or abnormal imaging (such as echocardiogram or chest x-ray)
- Symptomatic known or suspected thoracic aortic aneurysm:
- Signs and Symptoms may include:
- Abrupt onset of severe sharp or stabbing pain in the chest, back or abdomen
- Asymmetric blood pressure between limbs
- Acute chest or back pain and at high risk for aortic aneurysm and/or aortic syndrome (risk factors include hypertension, atherosclerosis, prior cardiac or aortic surgery, underlying aneurysm, or connective tissue disorder (e.g., Marfan syndrome, vascular form of Ehlers-Danlos syndrome, Loeys-Dietz syndrome))
- Signs and Symptoms may include:
Thoracic Aortic Syndromes(14,15)
- For suspected acute aortic syndrome (AAS) (such as aortic dissection, intramural hematoma and penetrating atherosclerotic ulcer) with any ONE of the following:
- Prior imaging (such as echocardiogram) is suggestive of AAS
- High risk patient for AAS and one sign/symptom concerning for AAS :
- High risk conditions:
- Marfan's syndrome or other connective tissue disease, family history of aortic disease, known aortic valve disease, recent aortic manipulation and/or known thoracic aortic aneurysm
- High risk conditions:
- Signs and symptoms concerning for AAS:
- Chest, back or abdominal pain described as abrupt onset, severe in intensity and/or ripping or tearing in quality
- Pulse deficit or systolic blood pressure differential
- Focal neurologic deficit with pain
- New heart murmur with pain
- Hypotension or shock
- Non-high-risk patient and two or more signs/symptoms concerning for AAS (see above)
- For follow-up of known aortic syndromes, including aortic dissection, intramural hematoma and penetrating atherosclerotic ulcer (frequency for follow up is as clinically indicated)
- Suspected vascular cause of dysphagia (from vascular compression of the esophagus) or expiratory wheezing (from vascular compression of the trachea/bronchus) with prior imaging that is indeterminate or abnormal.
Follow-Up of Known Thoracic Aortic Aneurysm
- Baseline study at diagnosis then every 6–24 months
- If there is a change in clinical status or cardiac exam, then imaging sooner than 6 months is indicated
Postoperative Follow-Up of Aortic Repair
- Follow-up thoracic endovascular aortic repair (TEVAR) at the following intervals(14,15):
- Baseline study at 1-month post-EVAR
- Annually thereafter if stable
- More frequent imaging (as clinically indicated) may be needed if there are complications or abnormal findings on surveillance imaging
- After 5 post-operative years without complications, continuing follow-up every 5 years should be considered
- Follow up after thoracic aorta open repair at the following intervals:
- At one-year post-repair
- Every 5 years thereafter
- If abnormal findings are seen on any surveillance imaging study, imaging is then done annually
Non-Aortic Vascular Disease
- Superior Vena Cava (SVC) syndrome when CTA/MRA are contraindicated or cannot be performed(17)
- SVC syndrome is a clinical diagnosis and may be suspected when there are signs of venous congestion in the upper body (such as shortness of breath, distended neck veins and facial/upper extremity edema)
- Neurogenic or venous thoracic outlet syndrome(18)
- Suspected pulmonary hypertension when other testing (echocardiogram or right heart catheterization) is suggestive of the diagnosis(19)
NOTE: Chest CT is NOT indicated for Pulmonary Embolism (PE) see Evolent Clinical Guideline 2020 for Chest CTA
Congenital Malformation
- Thoracic malformation on chest X-ray20
- Congenital Heart Disease with pulmonary hypertension21
- Malformations (such as pectus excavatum, pectus carinatum, scoliosis) in patients with cardiorespiratory symptoms for whom treatment is being considered
Transplants
Pre-Transplant
- Prior to solid organ transplantation (donor or recipient)
- For workup prior to Bone Marrow Transplant (BMT)
Post-Transplant23
- Routine surveillance of prior lung transplantation
- Concern for complication at any time following lung transplantation (CXR not required)
Chest Wall Pain and Injuries
- Non-traumatic chest wall pain with normal chest x-ray and or rib x-ray with any ONE of the following(24):
- Suspicion of malignancy
- Signs and symptoms of infection, such as fever, elevated inflammatory markers, known infection at other sites
- History of chest radiation or chest surgery
- Suspected chest wall injuries (including musculotendinous, costochondral cartilage, sternoclavicular joint, and manubriosternal joint injuries) with prior non-diagnostic or indeterminate imaging (such as x-ray or ultrasound) when imaging will potentially alter management
- Recent blunt trauma and suspected pleural effusion with recent chest x-ray(4)
Other Indications
- Pneumothorax on chest x-ray when imaging findings will change management
- Hemoptysis after x-ray completed(25)
- Vocal cord paralysis on endoscopic exam
- Phrenic nerve paralysis on diaphragm fluoroscopy (fluoroscopic) sniff test
- Evaluation of possible airway pathology with any ONE of the following:
- Indeterminate or abnormal prior imaging (such as X-ray, ultrasound, fluoroscopy)
- Clinical evidence suggesting airway obstruction (such as stridor, stertor, dyspnea on exertion)
- For evaluation of known or suspected tracheal stenosis(26)
- Large type IV hiatal hernia or diaphragmatic hernia (e.g. Bochdalek, Morgagni or Congenital)(27,28)
- For fever of unknown origin (temperature of ≥101 degrees for a minimum of 3 weeks) after all of the following has been completed and a source is not identified: complete blood count with differential, three sets of blood cultures, chest x-ray, complete metabolic panel, urinalysis, ESR, ANA, RA, serologic testing (EBV, EMV, HIV and hepatitis), tuberculin test and Abdomen and Pelvis CT or MRI(29)
Suspected Malignancy
- Further evaluation of one of the following identified on another imaging study:
- Lymphadenopathy
- Mediastinal mass(30)
- Lung mass not described as a nodule (generally > 3 cm)
- Pleural thickening, nodule or mass
- Chest wall mass(31)
NOTE: For any of the above: one follow-up study to ensure stability is indicated.
Additional follow-up studies indicated only if there is a change in the finding on repeat imaging
- For further evaluation of the following:
- Weight loss of ≥ 5% over 12 months AND signs and symptoms consistent with a source in the chest (such as a smoker with a cough) after initial Chest X-ray(32)
- Weight loss of ≥ 5% over 12 months when initial evaluation with Chest X-ray, age-appropriate cancer screening (such as colonoscopy and mammography), labs (including CBC, CMP, HbA1C, TSH, stool hemoccult, ESR/CRP, HIV, Hepatitis C) fail to identify a cause AND there is documented further decline in weight(32)
- Documentation of concern for malignancy (i.e. lymphoma) and any one of the following B symptoms:
- Fevers > 101°F
- Drenching night sweats
- Unexplained weight loss of > 10% body weight
- Gestational trophoblastic disease when hCG fails to decline appropriately following surgery
- Suspected paraneoplastic syndrome (including dermatomyositis) when appropriate workup has been done and there is a suspicion of malignancy(32)
- Evaluation for suspected thymoma screening in Myasthenia Gravis patients(33)
Known Malignancy
Initial Staging
- Chest CT is appropriate for initial staging of the majority of malignancies when either biopsy proven or suspected based on prior imaging.
Restaging
- Chest CT is indicated for restaging during active treatment (every 2-3 cycles of chemo or immunotherapy, following radiation and/or after surgery) for the majority of cancers
- Chest CT is indicated in addition to PET while on active treatment every 2-3 cycles of chemo or immunotherapy for the following: Ewing Sarcoma(34), Osteosarcoma (35), Hodgkin Lymphoma(36), Pediatric Aggressive Mature B-Cell Lymphomas(37), Pediatric Hodgkin Lymphoma(38), Soft Tissue Sarcoma(34) (if receiving systemic chemotherapy)
Recurrence
Chest CT is appropriate evaluation of a suspected recurrence for a patient with a known history of malignancy
Surveillance
Chest CT is indicated during surveillance for the following malignancies at the intervals defined below:
NOTE: For any patient with stage IV cancer (any type) that is either in remission or on a treatment break, chest CT is indicated every 3-6 months
- Adrenocortical Carcinoma: every 3-12 months for 5 years then as clinically indicated(39)
- Anal Carcinoma: every 3-6 months for 1-2 years, then every 6-12 months for an additional year(40)
- Biliary Tract Cancers (Ampullary Adenocarcinoma, Cholangiocarcinoma and Gallbladder): every 3-6 months for 2 years then every 6-12 months for up to 5 years then as clinically indicated(41,42)
- Bladder Cancer (Muscle Invasive OR Urothelial Carcinoma of the Upper Urinary Tract, Prostate or Urethra): every 3-6 months for 2 years, then annually for up to 5 years then as clinically indicated(43)
- Bone Tumors and Sarcomas (Chondrosarcoma, Chordoma, Giant Cell Tumor of Bone, Ewing Sarcoma, Soft Tissue Sarcoma, Osteosarcoma)(35)
- Every 3-6 months for 5 years, then annually for an additional 5 years, then as clinically indicated
- Colon Cancer(44):
- Stage II: every 6-12 months for 5 years, then as clinically indicated
- Stage III: every 3 months for 2 years, then every 6-12 months for 3 years, then as clinically indicated
- Esophageal and Esophagogastric Junction Cancers: every 3-6 months for 2 years, then annually for up to 5 years(45)
- Gastric Cancer: every 6 months for 2 years, then annually up to 5 years(46)
- Hepatocellular Carcinoma: every 3-6 months for 2 years, then every 6 months indefinitely(47)
- Lymphoma (Follicular, Diffuse Large B-Cell, Burkitt, Hodgkin, Marginal Zone, T-Cell) and
- Hairy Cell Leukemia: every 3-6 months for 2 years, then annually(36,48–50)
- Melanoma: Cutaneous (stage II or higher): every 3-12 months for 2 years then every 6-12 months for 3 years, then as clinically indicated(51)
- Melanoma: Uveal every 3-6 months for 5 years, then every 6-12 months for 5 additional years then as clinically indicated(52)
- Merkel Cell Carcinoma: every 3-6 months for 3 years, then every 6-12 months indefinitely (53)
- Mesothelioma (Pleural and Peritoneal): every 3-6 months for 5 years then annually until 10 years, then as clinically indicated (54,55)
- Neuroblastoma: every 3 months for 1 year, then every 6-12 months for 1year, then annually for 1 year, then as clinically indicated(56)
- Neuroendocrine Tumors: every 3-6 months for 5 years then every 6-12 months for 5 years, then as clinically indicated(39)
- Non-Small Cell Lung Cancer: every 3 months for 3 years, then every 6 months for 2 years, then annually(57)
- Occult Primary Tumors: follow indications based on how cancer is being treated (e.g. if treating as head and neck, defer to head and neck cancer guidance for all future requests). If tumor type is unclear: every 3-6 months for 2 years, then every 6-12 months for 3 years then annually.(58)
- Ovarian cancer: every 3-6 months for 2 years then every 6-12 months for 3 years, then as clinically indicated(59)
- Pancreatic cancer: every 3-6 months for 2 years, then every 6-12 months as clinically indicated(60)
- Penile cancer: Every 3 months for 1 year, then every 6-12 months for 3 years, then as clinically indicated(61)
- Prostate Cancer: as clinically indicated(62)
- Renal Cell Carcinoma(63):
- Stage I - annually for 5 years, then as clinically indicated
- Stage II and higher - every 3-6 months for 3 years, then annually for 2 years, then as clinically indicated
- Rectal Cancer(64):
- Stage II, III - every 6-12 months for 5 years, then as clinically indicated
- Small Bowel Adenocarcinoma: every 6-12 months for 5 years(65)
- Small Cell Lung Cancer: every 2 months for the first year, every 3-4 months for years 2 and 3 then every 6 months during years 4 and 5 then annually(66)
- Soft Tissue Sarcoma: every 3-6 months for 2 years, then every 6-12 months for 3 years, then annually as clinically indicated(34)
- Testicular cancer (Stage IIA and higher): every 3 months for 1 year, then every 6 months for 1 year then annually for 2 years(67)
- Thymoma and Thymic Carcinoma: every 3-6 months for 2 years, then annually for up to 10 years(68)
- Wilm's Tumor: every 3 months for 2 years then every 6 months for 2 years(69)
When a cancer is not listed above, Chest CT is not routinely a part of surveillance for that cancer in an asymptomatic patient. There would need to be a sign or symptom of recurrence to consider Chest CT.
When the timeframe above for routine surveillance has elapsed, there would need to be a sign or symptom of recurrence to consider Chest CT.
PREOPERATIVE OR POSTOPERATIVE ASSESSMENT
When not otherwise specified in the guideline:
Preoperative evaluation:
- Pre-operative evaluation for Electromagnetic Navigation Bronchoscopy(70) (this is a non-diagnostic CT)
- Imaging of the area requested is needed to develop a surgical plan
Postoperative evaluation:
- Known or suspected complications
- A clinical reason is provided how imaging may change management
NOTE: This section applies only within the first few months following surgery
FURTHER EVALUATION OF INDETERMINATE FINDINGS
Unless follow-up is specified within the guideline:
- For initial evaluation of an inconclusive finding on a prior imaging report that requires further clarification
- One follow-up exam of a prior indeterminate MR/CT finding to ensure no suspicious interval change has occurred. (No further surveillance unless specified as highly suspicious or change was found on last follow-up exam.)
IMAGING IN KNOWN GENETIC CONDITIONS
- Alpha-1 Anti-Trypsin Deficiency (AATD): at diagnosis(71)
- BAP1-TPDS (BAP-1 tumor predisposition syndrome): with clinical concerns for malignant mesothelioma(72)
- BHDS (Birt-Hogg-Dube): annually starting at age 20 (or earlier with family history of renal tumors diagnosed before age 30)(73)
- Cystic Fibrosis - chest CT every 2 years and as needed to assess for bronchiectasis(74)
- Li-Fraumeni (TP53): annually (75)
- Multiple Endocrine Neoplasia type 1 (MEN1): annually starting at age 8(39,76)
- Hereditary Paraganglioma-Pheochromocytoma (PGL/PCC) Syndrome (including SDHx mutations): every 2 years (including at diagnosis) AND MRI is contraindicated or cannot be performed(77)
- Tuberous Sclerosis: every 5 years OR more frequently for follow-up of known findings or symptoms(78)
- For other syndromes and rare diseases not otherwise addressed in the guideline, coverage is based on a case-by-case basis using societal guidance.
Combination Studies for Known Genetic Conditions
NOTE: When medical necessity is met for an individual study AND conscious sedation is required (such as for young pediatric patients or patients with significant developmental delay), the entire combination is indicated)
Chest CT and Brain/Abdomen/Pelvis MRI
- Multiple Endocrine Neoplasia Syndrome Type 1 (MEN-1)(39,76)
- Annually starting at age 8
- NOTE: every 3 years include Brain MRI
Neck/Chest/Abdomen/Pelvis CT
- Hereditary PGL/PCC Syndromes (including SDHx mutations): every 2 years (including at diagnosis) AND MRI is contraindicated or cannot be performed(63,77)
OTHER COMBINATION STUDIES WITH CHEST CT
NOTE: When medical necessity is met for an individual study AND conscious sedation is required (such as for young pediatric patients or patients with significant developmental delay), the entire combination is indicated)
Chest/Abdomen and Pelvis CT
- As numerous disease processes, including but not limited to malignancy, may affect the chest, abdomen and pelvis, this combination is indicated when the guideline criteria for BOTH Chest CT and Abdomen and Pelvis CT have been met
- Documentation of concern for malignancy (such as lymphoma) and any ONE of the following B symptoms:
- Fevers > 101◦ F
- Drenching night sweats
- Unexplained weight loss of >10% body weight
Chest/Abdomen CT
- Large type IV hiatal hernia or diaphragmatic hernia (e.g. Bochdalek, Morgagni or Congenital)
Chest CT/Abdomen and Pelvis CT and PET
- CT of the original sites of disease is indicated in addition to PET while on active treatment every 2-3 cycles of chemo or immunotherapy for the following: Hodgkin Lymphoma, Pediatric Aggressive Mature B-Cell Lymphomas, Pediatric Hodgkin Lymphoma
Neck/Chest/Abdomen/Pelvis CT
- Hereditary PGL/PCC (Paraganglioma/Pheochromocytoma) syndromes (including SDHx mutations): every 2 years (including at diagnosis) AND MRI is contraindicated or cannot be performed (63,77) Chest CT and PET
- Chest CT is indicated in addition to PET while on active treatment every 2-3 cycles of chemo or immunotherapy for the following: Ewing Sarcoma, Osteosarcoma, Soft Tissue Sarcoma (if receiving systemic chemotherapy)
Chest CTA (or MRA) and Chest CT
- When needed for clarification of vascular involvement from tumor
Neck/Chest CT
- Vocal cord immobility on endoscopic exam with concern for recurrent laryngeal nerve lesion
- Phrenic nerve paralysis on diaphragm fluoroscopy (fluoroscopic sniff test)
- Evaluation of possible airway pathology with any ONE of the following:
- Indeterminate or abnormal prior imaging (such as x-ray, ultrasound, fluoroscopy)
- Clinical evidence suggesting airway obstruction (such as stridor, stertor, dyspnea on exertion)
- For evaluation of known or suspected laryngeal, subglottic, or tracheal stenosis (26)
- Evaluation of dysphagia after appropriate prior work up including endoscopy (EGD) and/or fluoroscopic studies (such as modified barium swallow, biphasic Esophogram) is indeterminate or abnormal(11)
Sinus/Maxillofacial/Neck/Chest/Abdomen CT
- Advanced imaging for Granulomatosis with Polyangiitis (GPA) (Formally Wegener’s Granulomatosis) with ONE of the following (9):
- Suspected GPA based on clinical findings (such as biopsy results, lab testing including antineutrophil cytoplasmic antibodies (ANCA))
- Known GPA when imaging results of a specific anatomic area is needed to guide systemic therapy
Sinus/Chest/Abdomen/Pelvis CT and Brain MRI
- Prior to all types of Bone Marrow Transplant
Combination Studies for Malignancy for Initial Staging or Restaging
Unless otherwise specified in this guideline, indication for combination studies for malignancy for initial staging or restaging:
- Concurrent studies to include CT or MRI of any of the following areas as appropriate depending on the cancer: Brain, Neck, Chest, Abdomen, Pelvis, Cervical Spine, Thoracic Spine or Lumbar Spine
Rationale/Background
Suspected paraneoplastic syndromes with no established cancer diagnosis: laboratory evaluation and imaging.
The laboratory evaluation for paraneoplastic syndrome is complex. If the appropriate lab test results are suspicious for malignancy, imaging is indicated.
For SIADH (hyponatremia + increased urine osmolality), there is a high association with small cell lung cancer, therefore imaging typically starts with chest CT. If other symptoms suggest a different diagnosis other than small cell lung cancer, different imaging studies may be reasonable.
For hypercalcemia (high serum calcium, low-normal PTH, high PTHrP) it is reasonable to start with bone imaging followed by a more directed evaluation such as mammogram, chest, abdomen and pelvis imaging as appropriate.
For Cushing syndrome (hypokalemia, normal-high midnight serum ACTH NOT suppressed with dexamethasone) abdominal and chest imaging is reasonable. If dexamethasone suppression test DOES suppress ACTH, pituitary MRI is reasonable.
For hypoglycemia, labs drawn during a period of hypoglycemia (glucose < 55, typically a 72 hour fast) (insulin level, C-peptide and IGF-2:IGF-1 ratio) should be done to evaluate for an insulinoma. An elevated insulin level, elevated C-peptide and/or normal IGF-2:IGF-1 ratio warrant CT or MRI abdomen to look for insulinoma. A low insulin, low C-peptide and/or elevated IGF-2:IGF-1 ratio warrant chest and abdominal imaging.
When a paraneoplastic neurologic syndrome is suspected, nuclear and cytoplasmic antibody panels are often ordered to further identify specific tumor types. Results are needed prior to imaging. Because these tests are highly specific, if an antibody highly associated with a specific cancer is positive, then further imaging for that cancer is reasonable. For example, anti-Hu has a high association with SCLC and chest CT would be reasonable. Anti-MA2 has a high association with testicular cancer and testicular ultrasound would be a reasonable next step.
Contraindications and Preferred Studies
- Contraindications and reasons why a CT/CTA cannot be performed may include: impaired renal function, significant allergy to IV contrast, pregnancy (depending on trimester)
- Contraindications and reasons why an MRI/MRA cannot be performed may include: impaired renal function, claustrophobia, non-MRI compatible devices (such as non-compatible defibrillator or pacemaker), metallic fragments in a high-risk location, patient exceeds wight limit/dimensions of MRI machine
Table 1: Lung-RADS Assessment Categories(3)
*This table is reproduced without alteration or edit in accordance with provisions in a Creative Commons License. The full document and license information can be found here: Lung RADS | American College of Radiology (acr.org)
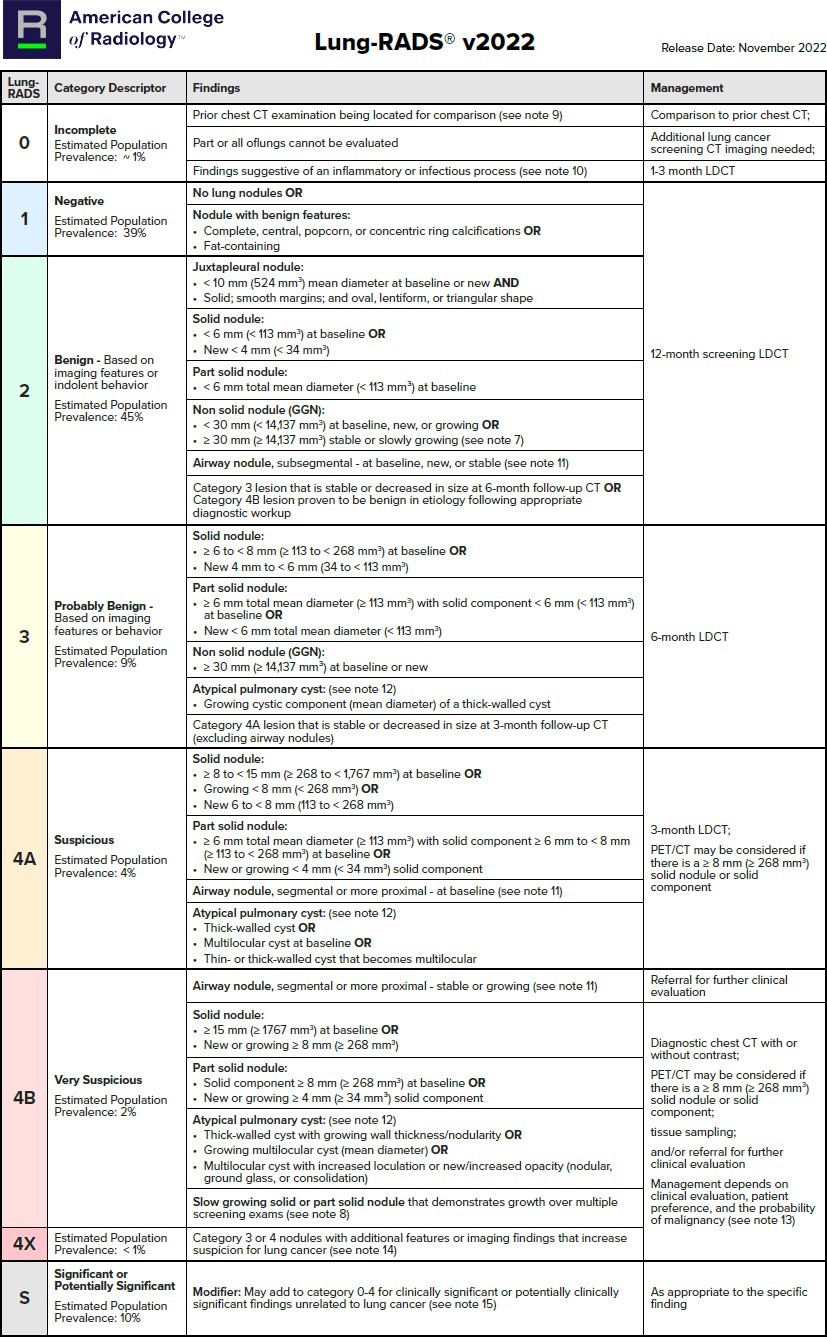
Table 2: 2017 Fleischner Society Guidelines for Management of Incidental Detected Pulmonary Nodules(1)
Fleischner Table
*This table is reproduced without alteration or edit.
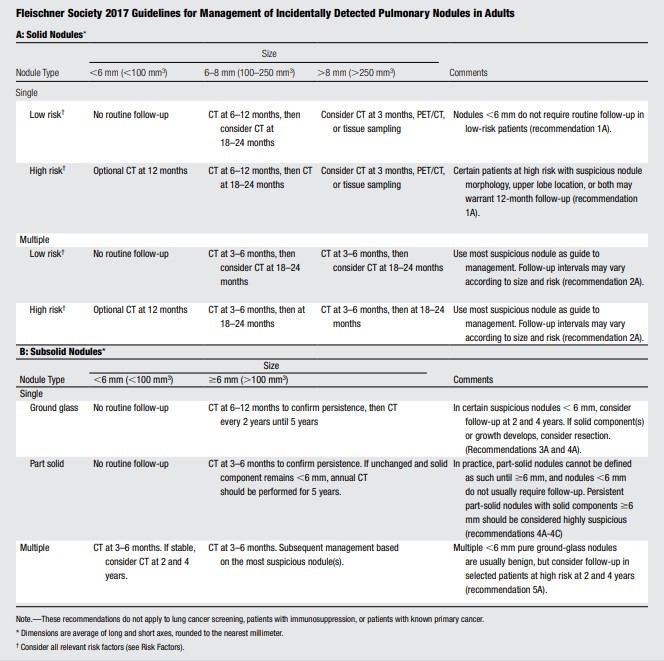
†There are multiple factors that may place an individual in the high-risk category such as smoking history and nodule characteristics. The designation of high risk may be assigned by the treating provider.
Cancer Risk Factors(1)
- Low Risk Factors
- Young age
- Less smoking
- Smaller nodule size
- Regular margins
- Location other than the upper lobe
- High Risk Factors
- Older age
- Heavy smoking
- Larger nodule size
- Irregular or spiculated margins
- Upper lobe location
SUMMARY OF EVIDENCE
Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017(1)
Study Design: This document presents the Fleischner Society guidelines for the management of incidental pulmonary nodules detected on CT images. The guidelines are based on a systematic review of the literature and expert consensus.
Target Population: The target population includes adults with incidentally detected pulmonary nodules on CT scans, excluding those with known primary cancers or who are
immunocompromised.
Key Factors: The guidelines provide recommendations for the follow-up and management of solid and subsolid nodules, with specific intervals for follow-up based on nodule size and patient risk factors. The document also discusses the importance of nodule morphology and location in assessing malignancy risk.
ACR Lung-RADS v2022: Assessment Categories and Management Recommendations(3)
Study Design: This document provides an update to the ACR Lung-RADS, which is a standardized system for reporting and managing screen-detected pulmonary nodules. The updates are based on a systematic review of the literature and expert consensus.
Target Population: The target population includes patients undergoing lung cancer screening with low-dose CT, particularly those at high risk for lung cancer.
Key Factors: The document introduces new classification criteria for atypical pulmonary cysts, juxtapleural nodules, and potentially infectious findings. It also provides updated management recommendations for these categories and clarifies the definition of nodule growth.
ACR Appropriateness Criteria® Diffuse Lung Disease (6)
Study Design: This document outlines the ACR Appropriateness Criteria for the evaluation of diffuse lung diseases (DLDs). The guidelines are based on an extensive review of current medical literature and expert opinion.
Target Population: The target population includes patients with suspected or confirmed diffuse lung diseases, such as interstitial lung disease.
Key Factors: The document provides guidelines for initial imaging, imaging during suspected acute exacerbations, and routine follow-up imaging. It emphasizes the importance of high-resolution CT (HRCT) and multidisciplinary discussions for accurate diagnosis and management.
ANALYSIS OF EVIDENCE
Analysis(1,3,6):
In summary, while all three articles highlight the importance of chest CT in diagnosing and managing lung diseases, they differ in their specific criteria for nodule classification, focus areas, and follow-up recommendations. The evidence presented in each article supports their respective guidelines and management strategies, providing a comprehensive approach to chest CT in clinical practice.
Shared Findings:
- Importance of Chest CT: All three articles emphasize the critical role of chest CT in diagnosing and managing lung diseases. Chest CT is highlighted as a superior imaging modality for detecting and characterizing lung nodules and diffuse lung diseases due to its high resolution and ability to provide detailed images of lung parenchyma.
- Nodule Management: The articles discuss the management of lung nodules, particularly the importance of follow-up and monitoring. They agree that the size and characteristics of nodules are crucial in determining the appropriate management strategy.
- Evidence-Based Guidelines: Each article presents evidence-based guidelines for the use of chest CT in different clinical scenarios. These guidelines are developed through systematic reviews of the literature and expert consensus.
References
- MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243. doi:10.1148/radiol.2017161659
- Bueno J, Lichtenberger JP, Rauch G, Carter BW. MR imaging of primary chest wall neoplasms. Topics in Magnetic Resonance Imaging. 2018;27(2):83-93. doi:10.1097/RMR.0000000000000164
- Christensen J, Prosper AE, Wu CC, et al. ACR Lung-RADS v2022: Assessment Categories and Management Recommendations. Journal of the American College of Radiology. 2024;21(3):473-488. doi:10.1016/j.jacr.2023.09.009
- Morris MF, Henry TS, Raptis CA, et al. ACR Appropriateness Criteria® Workup of Pleural Effusion or Pleural Disease. Journal of the American College of Radiology. 2024;21(6):S343-S352. doi:10.1016/j.jacr.2024.02.013
- Little BP, Gilman MD, Humphrey KL, et al. Outcome of recommendations for radiographic follow-up of pneumonia on outpatient chest radiography. American Journal of Roentgenology. 2014;202(1):54-59. doi:10.2214/AJR.13.10888
- Hobbs SB, Chung JH, Walker CM, et al. ACR Appropriateness Criteria® Diffuse Lung Disease. Journal of the American College of Radiology. 2021;18(11):S320-S329. doi:10.1016/j.jacr.2021.08.008
- ACR-STR. ACR–STR PRACTICE PARAMETER FOR THE PERFORMANCE OF HIGH-RESOLUTION COMPUTED TOMOGRAPHY (HRCT) OF THE LUNGS IN ADULTS. American College of Radiology. Published online 2020. Accessed January 8, 2025. https://www.acr.org/-/media/ACR/Files/Practice-Parameters/HRCT-Lungs.pdf
- Bokhari SRA, Zulfiqar H, Mansur A. Sarcoidosis. StatPearls. Published online June 25, 2025. https://www.ncbi.nlm.nih.gov/books/NBK430687/
- Watanabe R, Hashimoto M. Eosinophilic Granulomatosis with Polyangiitis: Latest Findings and Updated Treatment Recommendations. J Clin Med. 2023;12(18). doi:10.3390/jcm12185996
- Ravenel JG, Chung JH, Ackman JB, et al. ACR Appropriateness Criteria® Imaging of Possible Tuberculosis. Journal of the American College of Radiology. 2017;14(5):S160-S165. doi:10.1016/j.jacr.2017.02.022
- Levy AD, Carucci LR, Bartel TB, et al. ACR Appropriateness Criteria® Dysphagia. Journal of the American College of Radiology. 2019;16(5):S104-S115. doi:10.1016/j.jacr.2019.02.007
- Turner RD, Bothamley GH. Chronic cough and a normal chest X-ray - a simple systematic approach to exclude common causes before referral to secondary care: a retrospective cohort study. NPJ Prim Care Respir Med. 2016;26(1):15081. doi:10.1038/npjpcrm.2015.81
- Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve–related aortopathy: Full online-only version. J Thorac Cardiovasc Surg. 2018;156(2):e41-e74. doi:10.1016/j.jtcvs.2018.02.115
- Mazzolai L, Teixido-Tura G, Lanzi S, et al. 2024 ESC Guidelines for the management of peripheral arterial and aortic diseases. Eur Heart J. 2024;45(36):3538-3700. doi:10.1093/eurheartj/ehae179
- Isselbacher EM, Preventza O, Hamilton Black J, et al. 2022 ACC/AHA Guideline for the Diagnosis and Management of Aortic Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation. 2022;146(24):e334-e482. doi:10.1161/CIR.0000000000001106
- Mariscalco G, Debiec R, Elefteriades JA, Samani NJ, Murphy GJ. Systematic Review of Studies That Have Evaluated Screening Tests in Relatives of Patients Affected by Nonsyndromic Thoracic Aortic Disease. J Am Heart Assoc. 2018;7(15):e009302. doi:10.1161/JAHA.118.009302
- Azizi AH, Shafi I, Shah N, et al. Superior Vena Cava Syndrome. JACC Cardiovasc Interv. 2020;13(24):2896-2910. doi:10.1016/j.jcin.2020.08.038
- Zurkiya O, Ganguli S, Kalva SP, et al. ACR Appropriateness Criteria® Thoracic Outlet Syndrome. Journal of the American College of Radiology. 2020;17(5):S323-S334. doi:10.1016/j.jacr.2020.01.029
- Sharma M, Burns AT, Yap K, Prior DL. The role of imaging in pulmonary hypertension. Cardiovasc Diagn Ther. 2021;11(3):859-880. doi:10.21037/cdt-20-295
- Cancemi G, Distefano G, Vitaliti G, et al. Congenital Lung Malformations: A Pictorial Review of Imaging Findings and a Practical Guide for Diagnosis. Published online 2024. doi:10.3390/children
- Jone PN, Ivy DD, Hauck A, et al. Pulmonary Hypertension in Congenital Heart Disease: A Scientific Statement From the American Heart Association. Circ Heart Fail. 2023;16(7). doi:10.1161/HHF.0000000000000080
- Mak SM, Bhaludin BN, Naaseri S, Chiara F Di, Jordan S, Padley S. Imaging of congenital chest wall deformities. British Journal of Radiology. 2016;89(1061). oi:10.1259/bjr.20150595
- Kim SJ, Azour L, Hutchinson BD, et al. Imaging Course of Lung Transplantation: From Patient Selection to Postoperative Complications. RadioGraphics. 2021;41(4):1043-1063. doi:10.1148/rg.2021200173
- Stowell JT, Walker CM, Chung JH, et al. ACR Appropriateness Criteria® Nontraumatic Chest Wall Pain. Journal of the American College of Radiology. 2021;18(11):S394-S405. doi:10.1016/j.jacr.2021.08.004
- Olsen KM, Manouchehr-pour S, Donnelly EF, et al. ACR Appropriateness Criteria® Hemoptysis. Journal of the American College of Radiology. 2020;17(5):S148-S159. doi:10.1016/j.jacr.2020.01.043
- Little BP, Walker CM, Bang TJ, et al. ACR Appropriateness Criteria® Tracheobronchial Disease. Journal of the American College of Radiology. 2024;21(11):S518-S533. doi:10.1016/j.jacr.2024.08.015 Laracca GG, Spota A, Perretta S. Optimal workup for a hiatal hernia. Ann Laparosc Endosc Surg. 2021;6. doi:10.21037/ALES.2020.03.02
- American College of Radiolgoy. ACR Appropriateness Criteria® Hernia. American College of Radiolgoy . Published online 2022. https://acsearch.acr.org/docs/3158169/Narrative/
- Brown I, Finnigan NA. Fever of Unknown Origin. StatPearls. Published online August 14, 2023. https://www.ncbi.nlm.nih.gov/books/NBK532265/
- Ackman JB, Chung JH, Walker CM, et al. ACR Appropriateness Criteria® Imaging of Mediastinal Masses. Journal of the American College of Radiology. 2021;18(5):S37-S51. doi:10.1016/j.jacr.2021.01.007
- Mansour J, Raptis D, Bhalla S, et al. Diagnostic and Imaging Approaches to Chest Wall Lesions. RadioGraphics. 2022;42(2):359-378. doi:10.1148/rg.210095
- Thomas K, Gould M, Naeger D. Overview of the initial evaluation, diagnosis, and staging of patients with suspected lung cancer. UpToDate - Wolters Kluwer. April 2025. https://www.uptodate.com/contents/overview-of-the-initial-evaluation-diagnosis-and-staging-of-patients-with-suspected-lung-cancer
- Strange CD, Ahuja J, Shroff GS, Truong MT, Marom EM. Imaging Evaluation of Thymoma and Thymic Carcinoma. Front Oncol. 2022;11. doi:10.3389/fonc.2021.810419
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Soft Tissue Sarcoma Version 4.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bone Cancer Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hodgkin Lymphoma Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Pediatric Aggressive Mature B-Cell Lymphomas Version 2.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Pediatric Hodgkin Lymphoma Version 1.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Neuroendocrine and Adrenal Tumors Version 1.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Anal Carcinoma Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Ampullary Adenocarcinoma Version 2.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Biliary Tract Cancers Version 6.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/btc.pdf
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Bladder Cancer Version
- 6.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Esophageal and Esophagogastric Junction Cancers Version 5.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Gastric Cancer Version 5.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hepatocellular Carcinoma Version 4.2024. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for B-Cell Lymphomas Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for T-Cell Lymphomas Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hairy Cell Leukemia Version 1.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Cutaneous V.2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Melanoma: Uveal Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Merkel Cell Carcinoma Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Mesothelioma: Pleural Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Mesothelioma: Peritoneal Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Neuroblastoma Version 2.2024 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Non-Small Cell Lung Cancer Version 3.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Occult Primary (Cancer of Unknown Primary [CUP]) Version 2.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Ovarian Cancer Including Fallopian Tube Cancer and Primary Peritoneal Cancer Version 1.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Pancreatic Adenocarcinoma Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Penile Cancer Version 2.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Prostate Cancer Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Kidney Cancer Version 3.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Rectal Cancer V.1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Small Bowel Adenocarcinoma Version 2.2025. © National Comprehensive Cancer Network, Inc. 2024. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Small Cell Lung Cancer Version 4.2025 © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Testicular Cancer Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Thymomas and Thymic Carcinomas Version 1.2025. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Referenced with permission from the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Wilms Tumor (Nephroblastoma) Version 2.2024. © National Comprehensive Cancer Network, Inc. 2025. All rights reserved. To view the most recent and complete version of the guideline, go online to NCCN.org.
- Tan Y, Shen S jun, Wang C yun, Zhou Q juan, Jing QF. Comparative efficacy and safety of electromagnetic navigation bronchoscopy localization and CT- guided percutaneous localization in thoracoscopic resection of pulmonary nodules: a systematic review and meta-analysis. Published online September 30, 2022. doi:10.21203/rs.3.rs-2069587/v1
- Stoller JK, Hupertz V, Aboussouan LS. Alpha-1 Antitrypsin Deficiency. GeneReviews®. Published online June 1, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1519/
- Pilarski R, Byrne L, Carlo MI, Hanson H, Cebulla C, Abdel-Rahman M. BAP1 Tumor Predisposition Syndrome. GeneReviews®. Published online December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK390611/
- Sattler EC, Steinlein OK. Birt-Hogg-Dubé Syndrome. GeneReviews®. Published online December 5, 2024. https://www.ncbi.nlm.nih.gov/books/NBK1522/
- Savant A, Lyman B, Bojanowski C, Upadia J. Cystic Fibrosis. GeneReviews®. Published online August 8, 2024. https://www.ncbi.nlm.nih.gov/books/NBK1250/
- Schneider K, Zelley K, Nichols KE, Schwartz Levine A, Garber J. Li-Fraumeni Syndrome. GeneReviews®. Published online September 1, 2025. https://www.ncbi.nlm.nih.gov/books/NBK1311/
- Giusti F, Marini F, Brandi ML. Multiple Endocrine Neoplasia Type 1. GeneReviews®. Published online March 10, 2022. https://www.ncbi.nlm.nih.gov/books/NBK1538/
- Else T, Greenberg S, Fishbein L. Hereditary Paraganglioma-Pheochromocytoma Syndromes. GeneReviews®. Published online September 21, 2023. https://www.ncbi.nlm.nih.gov/books/NBK1548/
- Northrup H, Koenig MK, Pearson DA, Au KS. Tuberous Sclerosis Complex. GeneReviews®. Published online August 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK1220
Coding Section
| Code | Number | Description |
| CPT | 71250 | Computed tomography, thorax; without contrast material |
| 71260 | Computed tomography, thorax; with contrast material(s) | |
| 71270 | Computed tomography, thorax; without contrast material, followed by contrast material(s) and further sections | |
| 0722T | Quantitative Computed Tomography (CT) Tissue Characterization |
Procedure and diagnosis codes on Medical Policy documents are included only as a general reference tool for each policy. They may not be all-inclusive.
This medical policy was developed through consideration of peer-reviewed medical literature generally recognized by the relevant medical community, U.S. FDA approval status, nationally accepted standards of medical practice and accepted standards of medical practice in this community, and other nonaffiliated technology evaluation centers, reference to federal regulations, other plan medical policies, and accredited national guidelines.
"Current Procedural Terminology © American Medical Association. All Rights Reserved"
History From 2019 Forward
| 12/19/2025 | Annual review, updating policy for clarity and consistency. Removing lung cancer screening indication and CPT code 71271. Updating preoperative/postoperative, combination studies and genetic conditions. Adding statement to general information, airway indication, and high/low risk factors for Fleischner table. Also updating background, rationale, and references. |
| 12/02/2024 | Annual review, policy reformatted for clarity and consistency updating: Lung Cancer screening is consistent with Cancer society • Lung nodules sections was clarified for size and follow up studies • Infections and inflammation section added to incorporate indications within the GL that were alone and added in sarcoidosis • Reorganized the malignancy section to follow the abdomen GLs; for known malignancy Initial staging was broad, Restaging gave the situations not reasonable, and surveillance was each identified with timelines for acceptable studies • Genetic Syndromes and Rare Diseases was added/adjusted. • Combination Studies were expanded upon to coincide with other guidelines/combination studies. Also adding purpose, contraindications/preferred studies, rationale and references. |
| 11/30/2023 | Annual review, entire policy updated for consistency. Updated Covid information, clarified details on nudules seen in other imaging. Added tranplant imaging. |
| 12/16/2022 | Annual review, addition of single ventricle heart disease coverage criteria, No other changes. |
| 12/15/2021 |
Annual review updating existing information regarding Fleischner criteria and Lung Rads. Also adding section related to COVID 19. Updating description and references. |
| 12/10/2020 |
Updated coding with 2021 codes. No other changes. |
| 12/01/2020 |
Annual review, policy updated for clarity and also adding verbiage regarding low dose CT scanning previously in CAM 60130. Entire policy updated to encompass that addition. |
| 12/03/2019 |
New Policy |